PreTel Solutions

PreTel’s area sensor uterine electromyography (uEMG) monitoring solves the inability of existing technology to accurately identify independent contraction activity of individual regions of the uterus. Identification of this regional activity allows for improved care in the preterm labor patient, managing the progress of labor at time of delivery, and safety to the administration of induction therapy.
These improvements will avoid unnecessary C-Section deliveries, post-delivery maternal hemorrhage, and expensive neonatal intensive care nursery stays.
PreTel Monitoring Systems
PreTel pregnancy monitoring systems integrate the reporting of fetal heart rate (FHR), maternal heart rate (MHR), contraction activity and individual region activity into a portable patient wearable device. System solutions include interface to existing hospital central monitoring hardware and cloud access for home monitoring applications.
The PreTel pregnancy monitoring system improves both maternal and neonatal outcomes in the labor and delivery department, across hospital systems and throughout integrated health care networks.
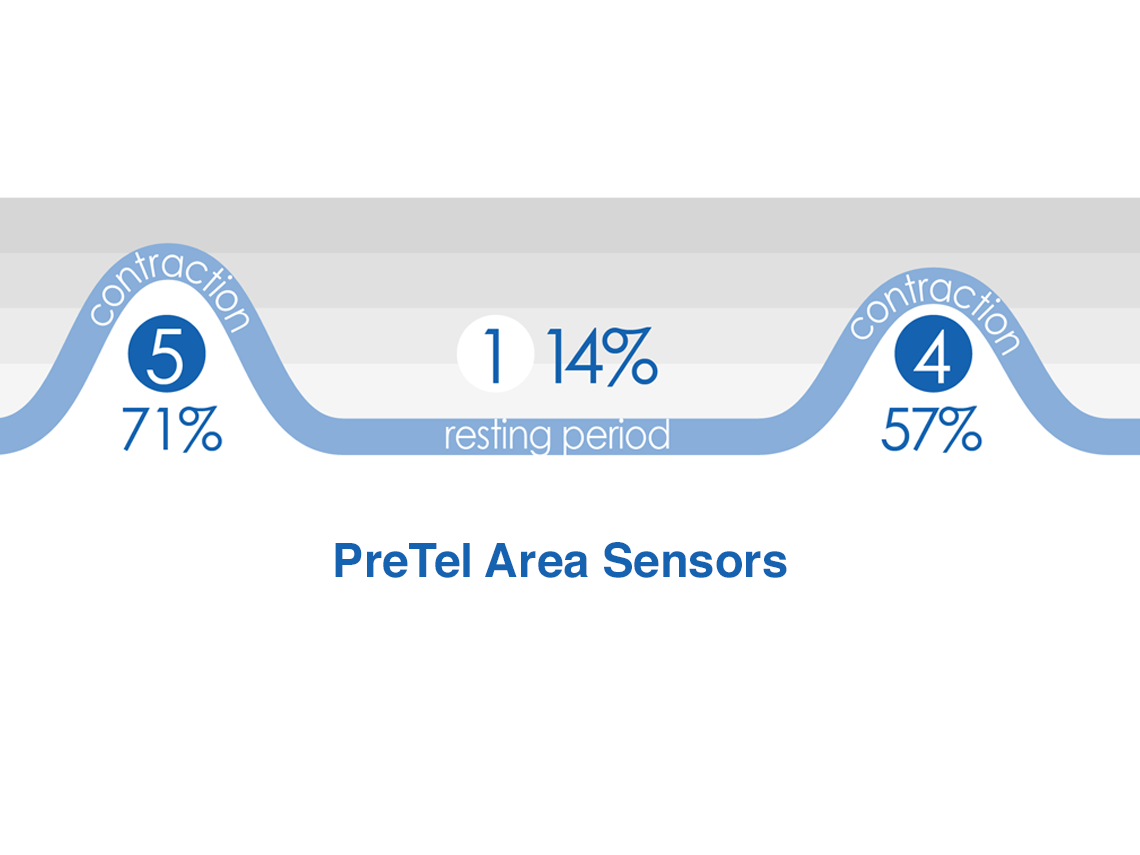
PreTel Area Sensors
Pregnancy monitoring impacts 3.7 Million births (US) improving both maternal and neonatal outcomes.
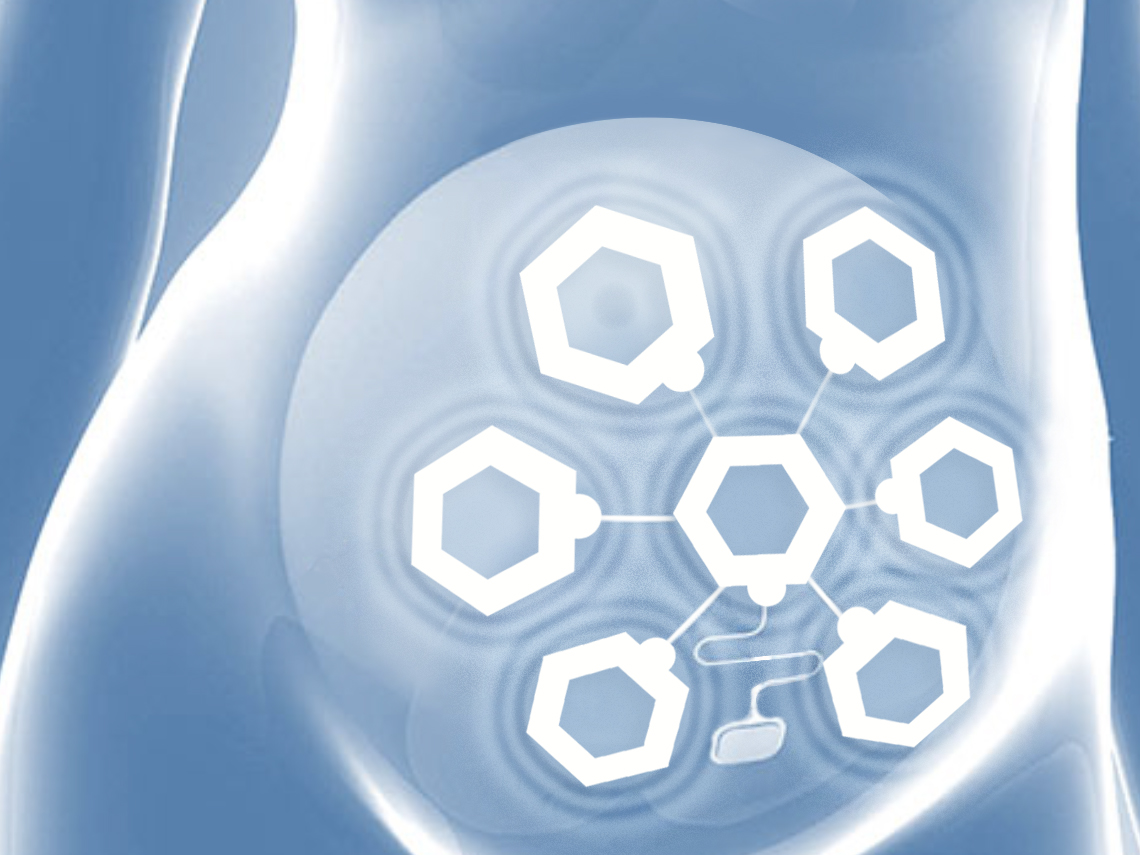
Regional uterine monitoring results in:
Supports preterm labor management
Evaluates therapy responseAccurate antepartum assessment
Differentiates false from true labor contractionsLabor progress information
Avoids unnecessary induction or C-sectionSafely conducts induction therapy
PreTel area sensors provide new information
Measure regional uterine synchronization
Indicates type of contractionEvaluates progress of labor
Increase/decrease of synchronizationAdditional Observations & Measurements